Collagen is an important protein component in animal tissue structure and in manufactured materials. It is used in applications as varied as leather, gelatin, and artificial skin.
Collagen fibers are hierarchical materials. The basic structural unit is an individual collagen monomer. A collagen ‘monomer’ contains three proteins arranged in a triple helix with nonhelical ends. In type 1 collagen, monomers are about 280-300 nm long and 1.5 nm in diameter. Other types have different monomer lengths. Three types of collagen form large fibrillar structures: Type I, found in skin, tendon, and bone; Type II, found in cartilage; and Type III found in Skin.
Five monomers assemble into one microfibril which is about 4 nm in diameter. The monomers align parallel to one another but are staggered so that the end of one monomer is offset by about 67 nm from its neighbor. Many microfibrils associate to form collagen fibrils; in skin, the diameter can range from 30 to 300 nm. Covalent cross links repeat at regular intervals along the fiber following the fixed stagger pattern and bind all of the molecules in the fiber together. This leads to the “D periodic” banding that is one of the characteristic features of fibrillar collagen examined by TEM (transmission electron microscopy). Because the monomer length is about 4.4 to 4.5D, a fiber contains gaps where the ends of linearly arranged monomers do not meet. On the other hand, regions of side-by-side overlap contain more protein than gap regions. In TEM images made using traditional negative staining, the gaps trap more of the stain and thus appear darker.
In AFM, with no staining, the D period is detected as a distinct height variation along the fibril’s length. In the example below, the height variation due to the D period was about 8% of the overall height of the fibril.
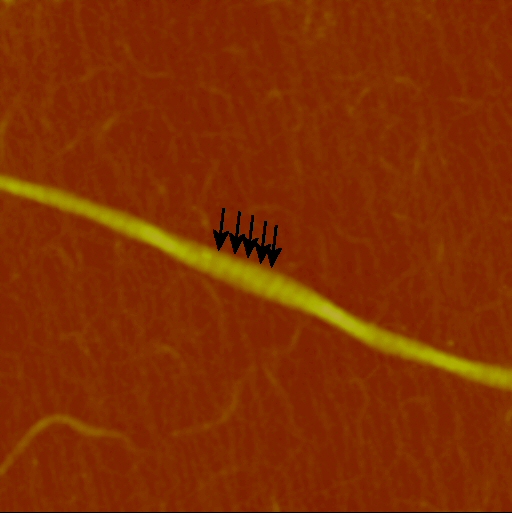
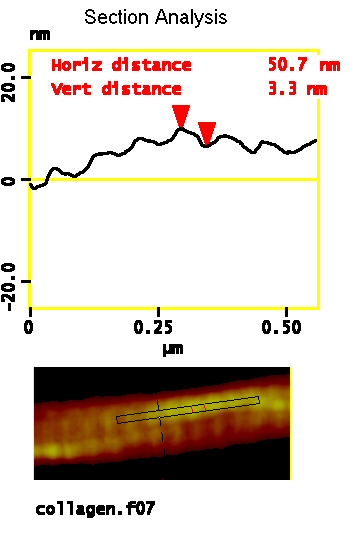
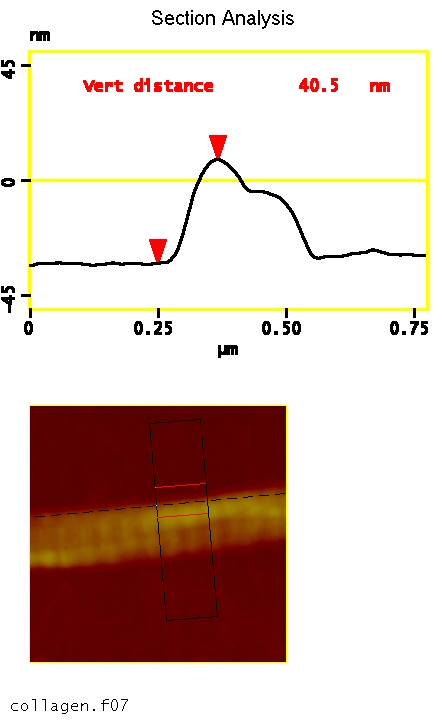
Due to the finite size of the AFM tip, the apparent width of nanoparticles such as collagen fibrils is larger than the actual width of the sample as prepared. Height measurements are not affected by the tip-width, so they provide reliable size information.
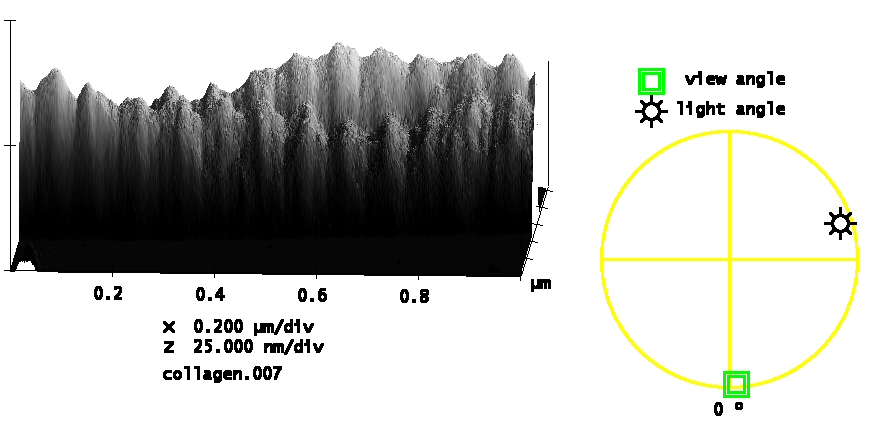
The asymmetry in height (tallest bump toward the left in each group) is consistent with the TEM banding pattern. Based on the prior assignment in the TEM literature, we state that the C-terminal direction of the fibril is toward the left.


